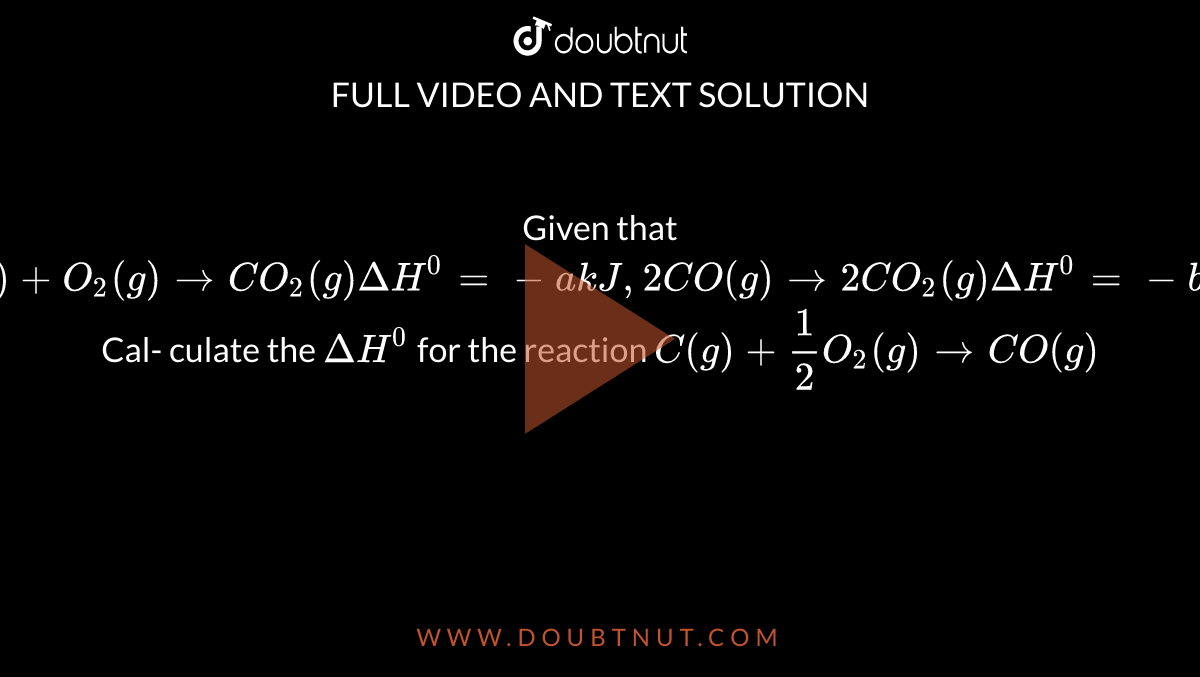
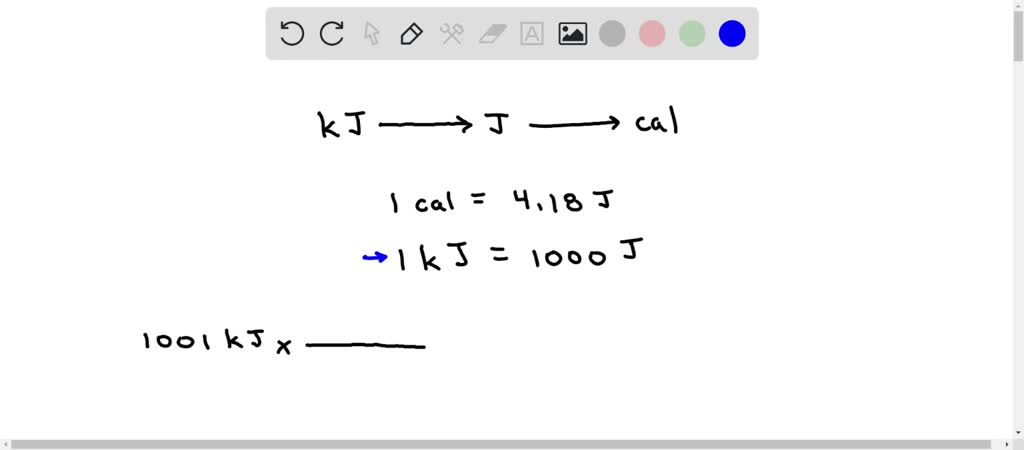
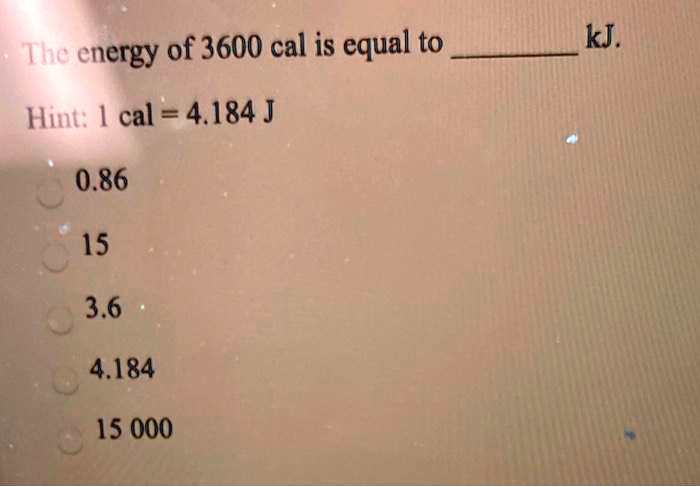![SOLVED: How many Joules of energy can candy bar with 148 Calories provide? Calorie kcab kcal 4.184 kJ cal = 4.184 ] kcal 1000 cal kJ = 1000 Answer: SOLVED: How many Joules of energy can candy bar with 148 Calories provide? Calorie kcab kcal 4.184 kJ cal = 4.184 ] kcal 1000 cal kJ = 1000 Answer:](https://cdn.numerade.com/ask_images/747afaea102d47f0901230d2751254f8.jpg)
SOLVED: How many Joules of energy can candy bar with 148 Calories provide? Calorie kcab kcal 4.184 kJ cal = 4.184 ] kcal 1000 cal kJ = 1000 Answer:
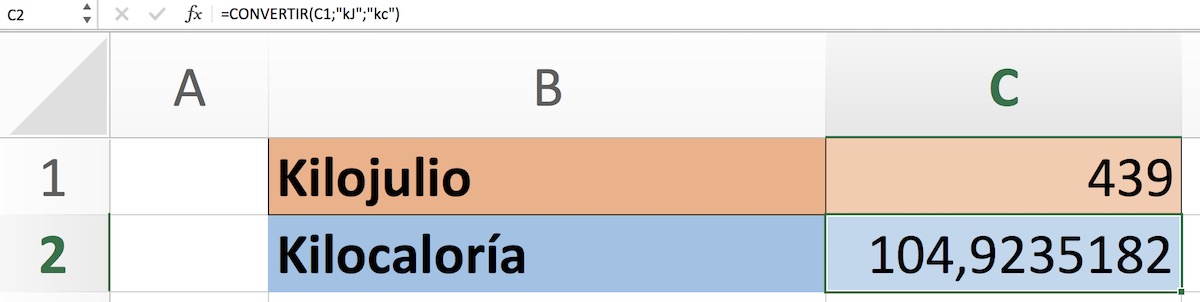
Curso Energía: pasado, presente y futuro Tema 1. Introducción Subtema 1.3 Combustibles usados por la humanidad a lo largo de l

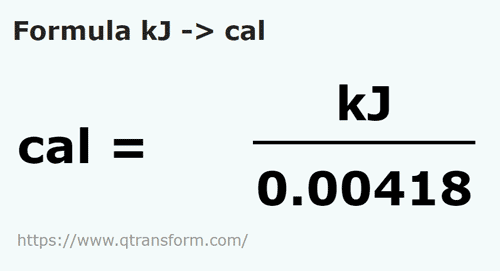
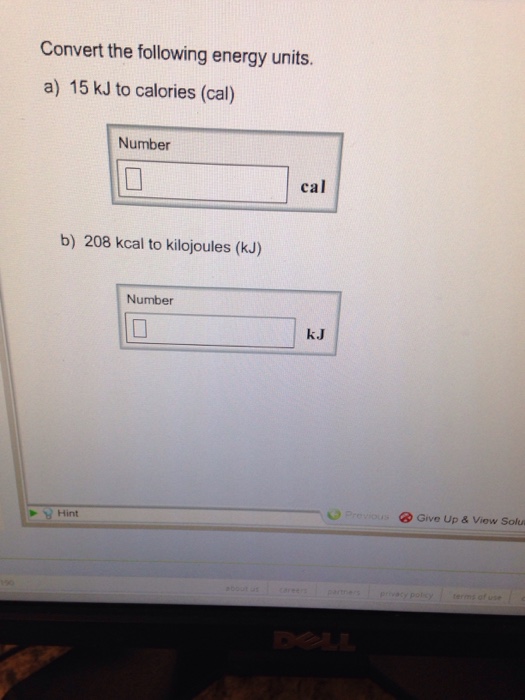
SOLVED: Convert 1.8 X 104 cal into kJ: (that is a little "c" cal) (1 cal = 4.184 J ) (little "c") 0443k @ B: 75 kJ O 643x106 kJ 0 0.7.S x107 kJ